Molar Mass of Beef Heart Myoglobin
| MB | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | MB, PVALB, myoglobgin, myoglobin, Myoglobin | ||||||||||||||||||||||||
| External IDs | OMIM: 160000 MGI: 96922 HomoloGene: 3916 GeneCards: MB | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Homo | Mouse | |||||||||||||||||||||||
| Entrez |
|
| |||||||||||||||||||||||
| Ensembl |
|
| |||||||||||||||||||||||
| UniProt |
|
| |||||||||||||||||||||||
| RefSeq (mRNA) |
|
| |||||||||||||||||||||||
| RefSeq (protein) |
|
| |||||||||||||||||||||||
| Location (UCSC) | Chr 22: 35.61 – 35.64 Mb | Chr 15: 76.ix – 76.93 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [four] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals.[5] [6] [vii] [8] [9] Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobin has a higher affinity for oxygen and does not have cooperative binding with oxygen similar hemoglobin does.[8] [10] In humans, myoglobin is only institute in the bloodstream afterwards musculus injury.[xi] [12] [13]
High concentrations of myoglobin in muscle cells allow organisms to concur their breath for a longer period of time. Diving mammals such equally whales and seals have muscles with particularly high abundance of myoglobin.[13] Myoglobin is plant in Type I muscle, Blazon II A, and Type Ii B, simply most texts consider myoglobin not to exist plant in smoothen musculus.[ citation needed ]
Myoglobin was the first protein to accept its three-dimensional structure revealed by X-ray crystallography.[14] This accomplishment was reported in 1958 past John Kendrew and associates.[15] For this discovery, Kendrew shared the 1962 Nobel Prize in chemistry with Max Perutz.[16] [17] Despite being one of the nearly studied proteins in biology, its physiological function is non yet conclusively established: mice genetically engineered to lack myoglobin can exist viable and fertile, but bear witness many cellular and physiological adaptations to overcome the loss. Through observing these changes in myoglobin-depleted mice, it is hypothesised that myoglobin function relates to increased oxygen send to muscle, and to oxygen storage; besides, it serves every bit a scavenger of reactive oxygen species.[18]
In humans, myoglobin is encoded by the MB factor.[nineteen]
Myoglobin tin take the forms oxymyoglobin (MbO2), carboxymyoglobin (MbCO), and metmyoglobin (met-Mb), analogously to hemoglobin taking the forms oxyhemoglobin (HbOii), carboxyhemoglobin (HbCO), and methemoglobin (met-Hb).[20]
Differences from hemoglobin [edit]
Like hemoglobin, myoglobin is a cytoplasmic protein that binds oxygen on a heme group. Information technology harbors only one globulin group, whereas hemoglobin has iv. Although its heme grouping is identical to those in Hb, Mb has a college analogousness for oxygen than does hemoglobin. This difference is related to its different office: whereas hemoglobin transports oxygen, myoglobin's office is to shop oxygen.
Office in cuisine [edit]
Myoglobin contains hemes, pigments responsible for the colour of carmine meat. The colour that meat takes is partly determined by the caste of oxidation of the myoglobin. In fresh meat the iron cantlet is in the ferrous (+2) oxidation state bound to an oxygen molecule (Oii). Meat cooked well done is brown considering the iron cantlet is now in the ferric (+3) oxidation land, having lost an electron. If meat has been exposed to nitrites, it will remain pink because the iron atom is bound to NO, nitric oxide (truthful of, eastward.g., corned beef or cured hams). Grilled meats can likewise accept on a reddish pink "smoke ring" that comes from the heme center binding to carbon monoxide.[21] Raw meat packed in a carbon monoxide atmosphere also shows this same pink "smoke ring" due to the same principles. Notably, the surface of this raw meat also displays the pink color, which is ordinarily associated in consumers' minds with fresh meat. This artificially induced pink color can persist, reportedly up to one year.[22] Hormel and Cargill (meat processing companies in the US) are both reported to employ this meat-packing process, and meat treated this way has been in the consumer marketplace since 2003.[23]
Role in disease [edit]
Myoglobin is released from damaged musculus tissue (rhabdomyolysis), which has very high concentrations of myoglobin.[24] The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may crusade astute kidney injury.[25] It is not the myoglobin itself that is toxic (it is a protoxin) only the ferrihemate portion that is dissociated from myoglobin in acidic environments (east.thou., acidic urine, lysosomes).
Myoglobin is a sensitive marker for musculus injury, making it a potential marker for heart assault in patients with chest hurting.[26] Notwithstanding, elevated myoglobin has depression specificity for acute myocardial infarction (AMI) and thus CK-MB, cardiac troponin, ECG, and clinical signs should be taken into account to brand the diagnosis.[27]
Structure and bonding [edit]
Myoglobin belongs to the globin superfamily of proteins, and as with other globins, consists of eight alpha helices connected by loops. Myoglobin contains 153 amino acids.[28]
Myoglobin contains a porphyrin band with an fe at its center. A proximal histidine group (His-93) is attached straight to fe, and a distal histidine grouping (His-64) hovers near the reverse face.[28] The distal imidazole is not bonded to the iron just is available to collaborate with the substrate O2. This interaction encourages the bounden of O2, simply non carbon monoxide (CO), which still binds near 240× more strongly than O2.
The bounden of O2 causes substantial structural change at the Fe center, which shrinks in radius and moves into the center of N4 pocket. O2-binding induces "spin-pairing": the five-coordinate ferrous deoxy form is loftier spin and the 6 coordinate oxy form is low spin and diamagnetic.[ citation needed ]
-

Molecular orbital clarification of Fe-O2 interaction in myoglobin.[29]
-
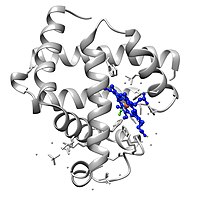
This is an image of an oxygenated myoglobin molecule. The image shows the structural change when oxygen is leap to the iron atom of the heme prosthetic group. The oxygen atoms are colored in dark-green, the atomic number 26 cantlet is colored in red, and the heme group is colored in blue.
Synthetic analogues [edit]
Many models of myoglobin have been synthesized as part of a broad interest in transition metal dioxygen complexes. A well known example is the watch contend porphyrin, which consists of a ferrous complex of a sterically bulky derivative of tetraphenylporphyrin.[xxx] In the presence of an imidazole ligand, this ferrous circuitous reversibly binds O2. The O2 substrate adopts a bent geometry, occupying the sixth position of the iron center. A key holding of this model is the ho-hum formation of the μ-oxo dimer, which is an inactive diferric state. In nature, such deactivation pathways are suppressed past poly peptide matrix that prevents close approach of the Fe-porphyrin assemblies.[31]
-

A picket-fence porphyrin complex of Fe, with axial coordination sites occupied by methylimidazole (green) and dioxygen. The R groups flank the Otwo-bounden site.
See also [edit]
- Cytoglobin
- Hemoglobin
- Hemoprotein
- Neuroglobin
- Phytoglobin
- Myoglobinuria - The presence of myoglobin in the urine
- Ischemia-reperfusion injury of the appendicular musculoskeletal system
References [edit]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000198125 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000018893 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Ordway GA, Garry DJ (Sep 2004). "Myoglobin: an essential hemoprotein in striated muscle". The Journal of Experimental Biology. 207 (Pt 20): 3441–6. doi:10.1242/jeb.01172. PMID 15339940.
- ^ Wick MR, Hornick JL (2011). "Immunohistology of Soft Tissue and Osseous Neoplasms". Diagnostic Immunohistochemistry. Elsevier. pp. 83–136. doi:10.1016/b978-1-4160-5766-6.00008-x. ISBN978-1-4160-5766-6.
Myoglobin is a 17.viii-kD protein that is establish in cardiac and skeletal musculus and that forms complexes with iron molecules.
- ^ Feher J (2017). "Oxygen and Carbon Dioxide Transport". Quantitative Human Physiology. Elsevier. pp. 656–664. doi:10.1016/b978-0-12-800883-6.00064-1. ISBN978-0-12-800883-6.
Highly oxidative muscle fibers contain a lot of myoglobin. It has two functions in muscle: information technology stores oxygen for use during heavy do, and information technology enhances diffusion through the cytosol past carrying the oxygen. Past bounden O2, myoglobin (Mb) provides a second deviating pathway for O2 through the cell cytosol.
- ^ a b Wilson MT, Reeder BJ (2006). "MYOGLOBIN". Encyclopedia of Respiratory Medicine. Elsevier. pp. 73–76. doi:10.1016/b0-12-370879-vi/00250-7. ISBN978-0-12-370879-3.
Myoglobin (Mb) is a heme-containing globular protein that is establish in abundance in myocyte cells of heart and skeletal musculus.
- ^ Boncyk JC (2007). "Perioperative Hypoxia". Complications in Anesthesia. Elsevier. pp. 193–199. doi:ten.1016/b978-1-4160-2215-2.50052-1. ISBN978-1-4160-2215-ii.
Myoglobin serves both as an O2 buffer and to store O2 in musculus. All known vertebrate myoglobins and β-hemoglobin subunits are like in structure, just myoglobin binds O2 more avidly at low Po2 (Fig. 47-5) considering it is a monomer (i.eastward., it does not undergo a significant conformational change with oxygenation). Thus, myoglobin remains fully saturated at O2 tensions between 15 and thirty mm Hg and unloads its O2 to the muscle mitochondria simply at very low O2 tensions.
- ^ Hardison RC (Dec 2012). "Evolution of Hemoglobin and Its Genes". Cold Bound Harb Perspect Med. ii (12): a011627. doi:10.1101/cshperspect.a011627. PMC3543078. PMID 23209182.
- ^ Chung MJ, Chocolate-brown DL (July 2018). "Diagnosis of acute myocardial infarction.". In Brownish DL (ed.). Cardiac Intensive Care-E-Book. pp. 91–98.e3. doi:x.1016/B978-0-323-52993-eight.00009-6. ISBN9780323529938.
Myoglobin is not specific for myocardial necrosis, however, peculiarly in the presence of skeletal muscle injury and renal insufficiency.
- ^ Sekhon N, Peacock WF (2019). "Biomarkers to Assistance in the Evaluation of Breast Pain". Biomarkers in Cardiovascular Disease. Elsevier. pp. 115–128. doi:10.1016/b978-0-323-54835-nine.00011-ix. ISBN978-0-323-54835-ix. S2CID 59548142.
myoglobin is non specific for the death of cardiac myocytes, and levels can be elevated in renal disease besides as impairment to skeletal musculus.
- ^ a b Nelson DL, Cox MM (2000). Lehninger Principles of Biochemistry (3rd ed.). New York: Worth Publishers. p. 206. ISBN0-7167-6203-X. (Google books link is the 2008 edition)
- ^ (U.S.) National Scientific discipline Foundation: Protein Data Bank Chronology (Jan. 21, 2004). Retrieved 3.17.2010
- ^ Kendrew JC, Bodo K, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC (Mar 1958). "A three-dimensional model of the myoglobin molecule obtained by 10-ray assay". Nature. 181 (4610): 662–half dozen. Bibcode:1958Natur.181..662K. doi:10.1038/181662a0. PMID 13517261. S2CID 4162786.
- ^ Stoddart, Charlotte (ane March 2022). "Structural biology: How proteins got their shut-up". Knowable Magazine. doi:10.1146/knowable-022822-1 . Retrieved 25 March 2022.
- ^ The Nobel Prize in Chemistry 1962
- ^ Garry DJ, Kanatous SB, Mammen PP (2007). "Molecular insights into the functional role of myoglobin". Advances in Experimental Medicine and Biology. 618: 181–93. doi:10.1007/978-0-387-75434-5_14. ISBN978-0-387-75433-8. PMID 18269197.
- ^ Akaboshi E (1985). "Cloning of the human myoglobin gene". Gene. 33 (iii): 241–9. doi:10.1016/0378-1119(85)90231-8. PMID 2989088.
- ^ Harvey JW (2008). "Iron Metabolism and Its Disorders". Clinical Biochemistry of Domestic Animals. Elsevier. pp. 259–285. doi:10.1016/b978-0-12-370491-7.00009-x. ISBN978-0-12-370491-7.
Myoglobin is an oxygen-binding protein located primarily in muscles. Myoglobin serves as a local oxygen reservoir that can temporarily provide oxygen when blood oxygen delivery is insufficient during periods of intense muscular activity. Iron within the heme group must be in the Fe+2 state to bind oxygen. If iron is oxidized to the Fe+iii land, metmyoglobin is formed.
- ^ McGee H (2004). On Food and Cooking: The Scientific discipline and Lore of the Kitchen. New York: Scribner. p. 148. ISBN0-684-80001-2.
- ^ Fraqueza MJ, Barreto AS (Sep 2011). "Gas mixtures approach to improve turkey meat shelf life under modified atmosphere packaging: the event of carbon monoxide". Poultry Science. 90 (9): 2076–84. doi:10.3382/ps.2011-01366. PMID 21844276.
- ^ "Meat companies defend use of carbon monoxide". Business organization. Minneapolis Star Tribune. Associated Press. 2007-ten-thirty. Archived from the original on 2013-12-25. Retrieved 2013-02-eleven .
- ^ Berridge BR, Van Vleet JF, Herman E (2013). "Cardiac, Vascular, and Skeletal Muscle Systems". Haschek and Rousseaux'south Handbook of Toxicologic Pathology. Elsevier. pp. 1567–1665. doi:ten.1016/b978-0-12-415759-0.00046-vii. ISBN978-0-12-415759-0.
Myoglobin is a depression molecular weight oxygen binding heme protein that is found exclusively in heart and skeletal muscle cells. In claret, myoglobin is spring primarily to plasma globulins, a complex which is filtered by the kidneys. If the plasma concentration exceeds the plasma binding chapters (i.5 mg/dl in humans), myoglobin begins to appear in the urine. Loftier concentrations of myoglobin can change the color of the urine to a dark carmine-brownish color.
- ^ Naka T, Jones D, Baldwin I, Fealy N, Bates Due south, Goehl H, Morgera S, Neumayer HH, Bellomo R (Apr 2005). "Myoglobin clearance by super loftier-flux hemofiltration in a case of severe rhabdomyolysis: a example report". Critical Care. nine (ii): R90-5. doi:ten.1186/cc3034. PMC1175920. PMID 15774055.
- ^ Weber Yard, Rau G, Madlener K, Elsaesser A, Bankovic D, Mitrovic V, Hamm C (Nov 2005). "Diagnostic utility of new immunoassays for the cardiac markers cTnI, myoglobin and CK-MB mass". Clinical Biochemistry. 38 (11): 1027–30. doi:ten.1016/j.clinbiochem.2005.07.011. PMID 16125162.
- ^ Dasgupta A, Wahed A (2014). "Cardiac Markers". Clinical Chemistry, Immunology and Laboratory Quality Command. Elsevier. pp. 127–144. doi:x.1016/b978-0-12-407821-five.00008-5. ISBN978-0-12-407821-5.
Myoglobin is a heme protein plant in both skeletal and cardiac muscle. Myoglobin is typically released in the circulation equally early as one h after myocardial infarction,... Myoglobin has poor clinical specificity due to the presence of large quantities of myoglobin in skeletal muscle. Some studies propose calculation the myoglobin exam to the troponin I test in lodge to meliorate diagnostic value [four]. Myoglobin, existence a small protein, is excreted in urine, and a loftier level of serum myoglobin is encountered in patients with acute renal failure (uremic syndrome). Acute renal failure is also a complication of rhabdomyolysis, ...
- ^ a b Universal protein resource accession number P02144 at UniProt.
- ^ Drago RS (1980). "Costless radical reactions of transition metal systems". Coordination Chemical science Reviews. 32 (2): 97–110. doi:10.1016/S0010-8545(00)80372-0.
- ^ Collman JP, Brauman JI, Halbert TR, Suslick KS (October 1976). "Nature of O2 and CO binding to metalloporphyrins and heme proteins". Proceedings of the National Academy of Sciences of the United States of America. 73 (10): 3333–7. Bibcode:1976PNAS...73.3333C. doi:10.1073/pnas.73.10.3333. PMC431107. PMID 1068445.
- ^ Lippard SJ, Berg JM (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: Academy Scientific discipline Books. ISBN0-935702-73-3.
Farther reading [edit]
- Collman JP, Boulatov R, Sunderland CJ, Fu L (February 2004). "Functional analogues of cytochrome c oxidase, myoglobin, and hemoglobin". Chemical Reviews. 104 (2): 561–88. doi:10.1021/cr0206059. PMID 14871135.
- Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT (Dec 2004). "The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human being pathology". Antioxidants & Redox Signaling. six (6): 954–66. doi:10.1089/ars.2004.6.954. PMID 15548893.
- Schlieper 1000, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flögel U, Gödecke A, Schrader J (April 2004). "Accommodation of the myoglobin knockout mouse to hypoxic stress". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 286 (4): R786-92. doi:10.1152/ajpregu.00043.2003. PMID 14656764. S2CID 24831969.
- Takano T (Mar 1977). "Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale". Periodical of Molecular Biology. 110 (iii): 569–84. doi:10.1016/S0022-2836(77)80112-5. PMID 845960.
- Roy A, Sen Due south, Chakraborti As (Feb 2004). "In vitro nonenzymatic glycation enhances the part of myoglobin as a source of oxidative stress". Complimentary Radical Inquiry. 38 (2): 139–46. doi:10.1080/10715160310001638038. PMID 15104207. S2CID 11631439.
- Stewart JM, Blakely JA, Karpowicz PA, Kalanxhi E, Thatcher BJ, Martin BM (Mar 2004). "Unusually weak oxygen binding, physical backdrop, partial sequence, autoxidation rate and a potential phosphorylation site of beluga whale (Delphinapterus leucas) myoglobin". Comparative Biochemistry and Physiology B. 137 (3): 401–12. doi:x.1016/j.cbpc.2004.01.007. PMID 15050527.
- Wu Grand, Wainwright LM, Poole RK (2003). Microbial globins. Advances in Microbial Physiology. Vol. 47. pp. 255–310. doi:ten.1016/S0065-2911(03)47005-7. ISBN9780120277476. PMID 14560666.
- Mirceta S, Signore AV, Burns JM, Cossins AR, Campbell KL, Berenbrink Yard (Jun 2013). "Evolution of mammalian diving capacity traced by myoglobin net surface charge". Science. 340 (6138): 1234192. doi:x.1126/scientific discipline.1234192. PMID 23766330. S2CID 9644255. . Also see Proteopedia article about this finding
External links [edit]
- Online Mendelian Inheritance in Man (OMIM): 160000 human genetics
- The Myoglobin Poly peptide
- RCSB PDB featured molecule
- "Which Cutting Is Older? (It's a Trick Question)", The New York Times, February 21, 2006 article regarding meat manufacture use of carbon monoxide to keep meat looking crimson.
- "Stores React to Meat Reports", The New York Times, March 1, 2006 commodity on the use of carbon monoxide to make meat announced fresh.
- Overview of all the structural data available in the PDB for UniProt: P02144 (Man Myoglobin) at the PDBe-KB.
Source: https://en.wikipedia.org/wiki/Myoglobin





0 Response to "Molar Mass of Beef Heart Myoglobin"
Post a Comment